The interface between a gas and a solid is a remarkable environment for new investigations. Lots of fascinating chemistry takes place there, including catalysis. Catalysis is acceleration of a chemical reaction that is caused by an element like platinum that remains unchanged by a chemical reaction. For instance platinum catalyzes the transformation of carbon monoxide (CO) into carbon dioxide (CO2) in automobile catalytic converters. A better understanding of catalysis could improve the efficiency of manufacturing important chemicals as well as expanding our fundamental knowledge of chemistry.
The challenge for Fellow David Nesbitt and his group is figuring out how catalysis works at the molecular level. Such an understanding requires a detailed understanding of the interface between a gas and a solid.
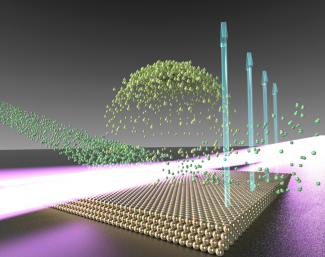
Former research associate Joseph Roscioli, former graduate student Dan Bell, graduate student Dan Nelson, and Nesbitt came up with a nifty method for studying one gas/solid interface. They investigated the interface between a gas (hydrochloric acid, or HCl) and a solid gold-nanocrystal surface on mica. They fired a supersonic jet of cold HCl molecules (about 1–2 K) at a hot flat surface (about 500 K). The cold jet of gas traveled way faster than a speeding bullet before crashing into or bouncing off the surface.
For the experiment, the group shined an ultraviolet laser about 100 microns (millionths of a meter) above the gold surface. When the laser tickled the supersonic HCl molecules, the molecules lost an electron and became positively charged. This gentle process didn’t affect the molecule’s speed. However, it allowed the researchers to use an electric field to coax the ions into going in a particular direction. The electric field also accelerated the charged molecules, or ions, so that when they struck a metal plate, they hit it with sufficient energy to liberate 1–10 million electrons.
When the electrons hit a phosphor screen, they made sparks of light, which were recorded by a camera. The results of 10,000–20,000 laser pulses were then fed into a computer. The computer created a velocity map of the ions, which allowed the researchers to determine the location, relative speed, and temperature of the ions after they scattered off the gold surface.
The researchers discovered that about 70% of the HCl molecules “swam” around on the gold surface before lifting back off — at the same temperature as the gold surface (500 K). The remaining HCl molecules immediately bounced off the surface rotating furiously at a temperature of 1000 K.
In the future, the Nesbitt group plans to coat its gold surface with sulfur atoms, creating a forest-like surface. Next the group will chemically engineer the tops of the “trees” on this surface and investigate chemical reactions with carbon-containing molecules. The researchers hope to engineer collision experiments with various different molecules.